The Drug Supply Chain Security Act – DSCSA (Title II, Drug Quality and Security Act, 2013) –has been signed into law and represents significant changes for U.S. pharmaceutical manufacturers. Full implementation of the Act is to be phased in over the next 10 years, with several modifications to packaging standards in the pharmaceutical industry expected. The next pivotal deadline, November, 2018, is approaching, requiring all pharmaceutical manufacturers to print unique product identification codes on all Rx units of sale, and any homogenous cases distributed domestically.
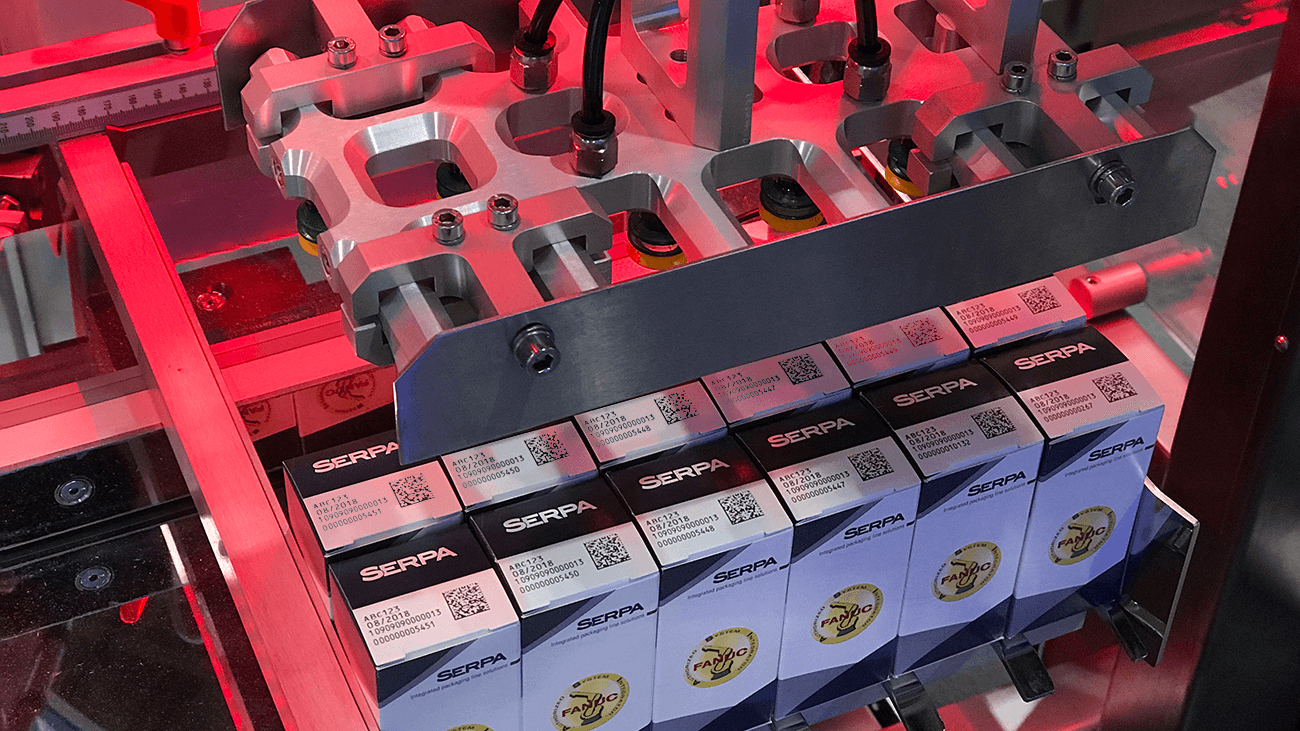
With just over one year left to be compliant, the U.S. pharmaceutical industry is at varied levels of preparation for the upcoming serialization mandates. Some major pharmaceutical and biopharmaceutical companies have gotten a leg up on others, already serializing some of their lines, or piloting with trading partners. Unfortunately, though, a majority of the large and mid-sized pharma corporations are just now in the beginning phases of drafting RFPs and strategy development. And with a typical timeline of 24 to 36 months to develop, implement, and pilot an operative strategy via a viable trading partner connection, many of these companies will have a difficult time complying by the deadline. Serpa, a world leader in packaging solutions, is providing a semi-automated case packing solution with an incredibly short lead time that can help pharmaceutical customers meet the deadline for serialization.
Incorporating years of extensive knowledge and expertise of both fully and semi-automated case packers, Serpa developed this machine as a lower cost option for those unable to afford a fully automated case packer. With its ModPack modules, customers use only those modules necessary to complete their respective projects, and the universal camera mount allows ModPack compatibility with all serialization and vision systems. The flexible, modular design of this unit allows for fast, efficient change overs, and the labeler “swings” open for easy operator and maintenance access to the load station, or the front and back of the labeler. It is the most compact footprint available on the market today, with unique collators designed to handle bottles, cartons & bundles.
The Serpa semi-automated case packing solution is the ideal choice for fast and reliable implementation, with quick lead times to meet customer serialization project deadlines and comply with the DSCSA packaging deadline of November, 2018.
Serpa Packaging provides an extensive array of custom solutions for the Pharmaceutical and Medical Deviceindustries. To learn more about their innovative products and more about the DSCSA changes to pharmaceutical packaging standards, visit https://www.serpapackaging.com/products/pharma/.

